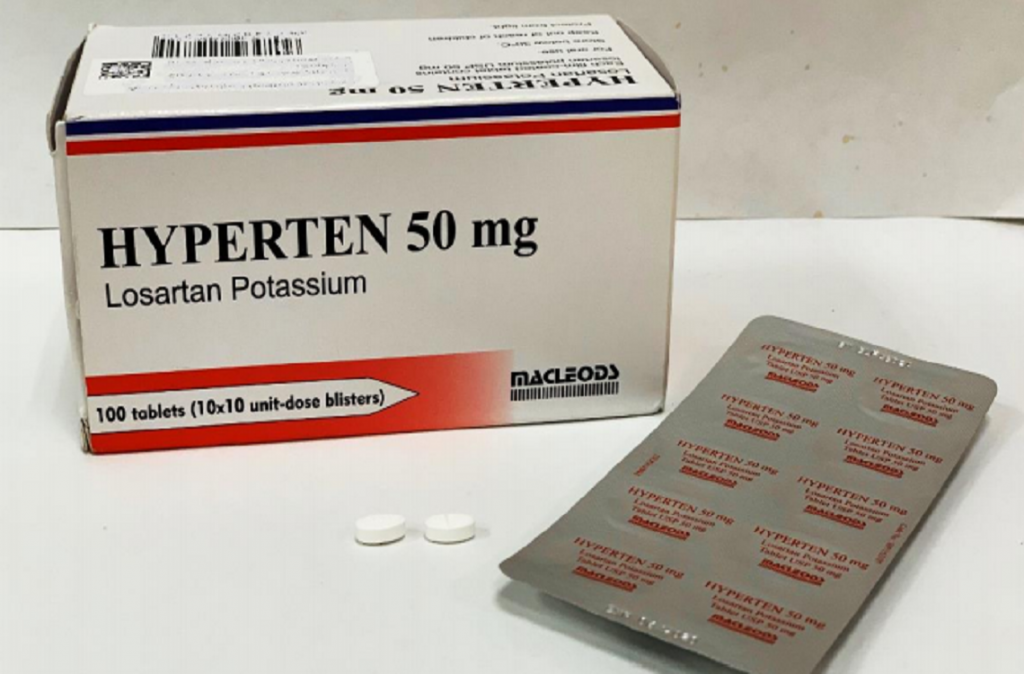
Recently, three brands of high blood pressure drugs have been recalled by Health Sciences Authority (HSA). They are Hyperten, Losagen and Losartas for both 50mg and 100mg tablets that contain cancer-causing toxins.
The affected medicines were manufactured by Hetero Labs Limited. According to HSA’s Press Release, the amount of nitrosamine impurity (NMBA) found in the medicines were higher than the acceptable level. Consequently, it will take a toll on a patient’s health who has been taking them for a long period.
Generally, losartan belongs to a class of medicines called angiotensin II receptor blockers (ARBs). It acts to treat high blood pressure, which is also known as hypertension.
Previous Investigation
Since February 2019, the company has been recalling all the losartan medicines to test for the presence of new NMBA impurity.
On March 21, the result stated that only three brands mentioned above were contaminated. The other seven brands are safe to consume and sold on the market.
Advice from the Experts
To date, more than 137,000 patients are taking the three recalled brands of high blood pressure medicines. Meanwhile, about 130,000 patients have been prescribed Losartas at both public hospitals and polyclinics.
Professor Ding Zee Pin, Cardiologist in the National Heart Centre Singapore and HSA’s Expert Panel on Nitrosamines said:
There is no immediate health risk associated with taking the affected medicines, hence, it is not advisable for parents to stop or change treatment on their own.
He further added that sudden stopping of the medicines might impose a greater risk of poor control of high blood pressure.
The Ministry of Health (MOH) advised the healthcare professional to review the patient’s medicine and provide them with a suitable replacement plan.
For those patients whose appointment are scheduled before July 1 should wait until then to see the doctor and get the alternative medicines. Patients whose appointments are arranged after July 1 will be contacted for an earlier consultation, mentioned MOH.
It also announced that there will be no charges for the additional consultations or tests to assess suitability for a switch in medicine. Patients will receive the refund if they return the affected losartan medications at public healthcare institutions.
Adequate Supply of High Blood Pressure Medication
MOH also mentioned that the public healthcare sector has ordered an abundant amount of unaffected brands of losartan. The additional supplies will arrive progressively from a few weeks’ time. Meanwhile, importers will set aside extra supplies for private healthcare providers, and the doctors are advised to prescribe the medication on a one-month basis.
MOH also stressed that,
This is to make sure every patient in all settings will promptly receive the medication they need. This may be the practice for the next six months.
Besides, HSA is currently working with companies and international regulatory agencies to sort out the cause of contamination. Proper measure and actions will be formulated to address the issue.
HSA also urges the companies to make the necessary changes to their manufacturing process. It is to make sure they will comply with the international standards to guarantee the quality of the products.
For patients who wish to inquire further about this matter, please contact the HSA hotline at 68663538 (telephone) or email at contact_hprg@hsa.gov.sg.
For more information about parenting and family, please visit Mamahood.com.sg.